Background:
Isocitrate dehydrogenase enzymes catalyze the conversion of isocitrate to alpha-ketoglutarate. Mutated IDH enzymes generate the "oncometabolite" 2-hydroxyglutarate (2-HG), which inhibits TET2 function.
IDH2 mutations have been reported in 9% to 19% of acute myeloid leukemia (AML) cases. Inhibition of the mutant IDH2 enzyme led to decreased 2-HG levels and induced myeloid differentiation in IDH2 mutant AML.
Enasidenib, a small-molecule inhibitor of mutant IDH2, was approved in 2017, after a successful phase I/II trial showing an overall response rate (ORR) of 40.3% in relapsed/refractory (R/R) disease, with 19.3% of patients achieving complete remission (CR). A Phase III trial is currently ongoing. It targets the mutant IDH2 variants R140Q, R172S, and R172K; at an approved dose of 100 mg oral daily dose.
At higher doses the drug was less tolerated, however, few subjects received dose modification at the 650 mg daily dose group; these events did not qualify as a dose-limiting toxicity (DLT).
In this descriptive study, we are reporting a case series of R/R AML, with an IDH2 mutation, that are treated with escalated dose enasidenib.
Method:
This case series is based on retrospective observations of patients with R/R IDH2 mutant AML since January 2017, who are treated with enasidenib. We are reporting two cases treated with an escalated dose of 200 mg daily.
We included patients with intermediate to poor-risk AML, who received at least one prior line of therapy, started on standard dose enasidenib (100 mg daily) for a minimum of 6 months and followed until disease relapse or death. We excluded patients who are primarily resistant to enasidenib (AML risk stratification and response evaluation by ELN-Leukemia NET.
IDH2 mutations were identified by a local diagnostic laboratory that is regulated under CLIA through PCR which is validated to detect 10% or more mutant allele frequency. To quantify IDH2 variant frequency, confirmatory testing was performed via ARUP next-generation sequencing panel (NGS).
Results:
Here we report the descriptive outcomes of 2 cases from a single institution, who were treated with escalated dose enasidenib (200 mg oral daily) for R/R AML with an IDH2 mutation.
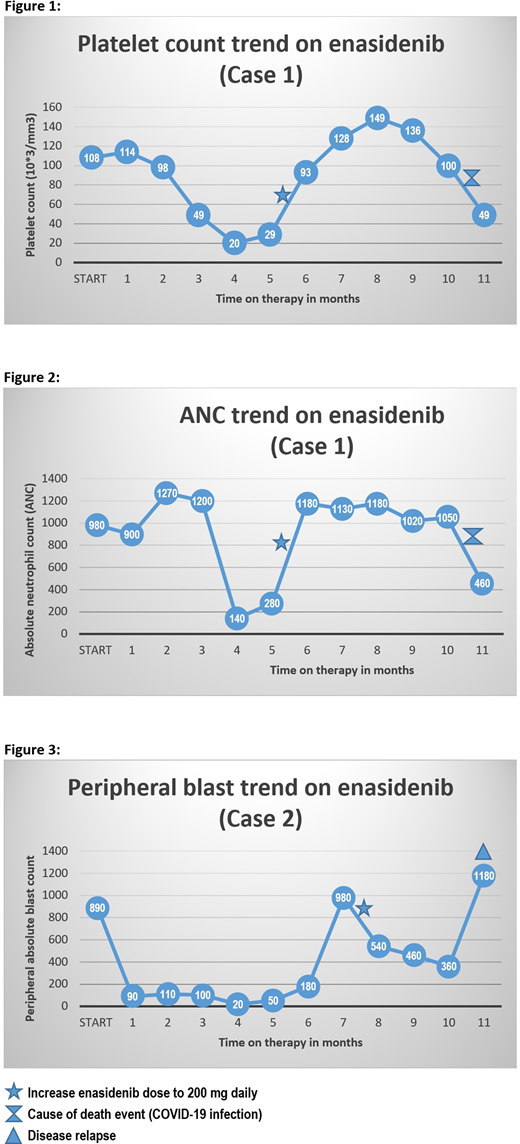
The first case describes a 65-year-old female diagnosed with poor-risk AML in the setting of pancytopenia with bone marrow evaluation consistent with the background of erythroid and granulocytic dysplasia, with normal karyotype analysis. The patient has treated with induction chemotherapy and 2 cycles consolidation that was complicated with bacterial infections, prolonged hospitalization, and profound deconditioning led to stopping treatment. After 18 months of surveillance, repeat bone marrow evaluation for new-onset pancytopenia revealed relapsed AML with evidence of IDH2 mutation at codon 140, with a variant frequency of 36.9% by NGS. The case was started on enasidenib at 100 mg oral daily dose. After 6 months of therapy, the patient continued to be cytopenic, repeated bone marrow evaluation showed residual AML with IDH2 variant frequency of 27.6%. The decision was made to increase the enasidenib dose to 200 mg daily. With follow up for another 5 months on the escalated dose, the patient achieved hematologic complete response (hCR) with normalization of peripheral platelet count and ANC. [Figures 1-2] The patient maintained hCR for another 5 months on 200 mg enasidenib daily until she died from COVID-19 infection.
The second case describes a 59-year-old female with poor-risk AML in the setting of leukocytosis, with bone marrow evaluation consistent with the background of megakaryocytic dysplasia and karyotype analysis positive for monosomy 7. The patient was treated with induction chemotherapy and failed to respond. Repeat bone marrow evaluation showed primary refractory disease with positive IDH2 mutation at codon 140 with a variant frequency of 40.7%. Treatment with 100 mg enasidenib was started. The patient maintained a partial response for 6 months, then peripheral blasts counts started to gradually increase. The decision was made to increase enasidenib dose to 200 mg daily, which improved peripheral blasts within one month of escalated dose therapy. [Figure 3] The patient maintained a response to therapy for another 3 months before she relapsed.
Conclusion:
Our limited data showed that initial response to standard dose enasidenib could potentially be optimized by dose escalation to 200 mg daily.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal